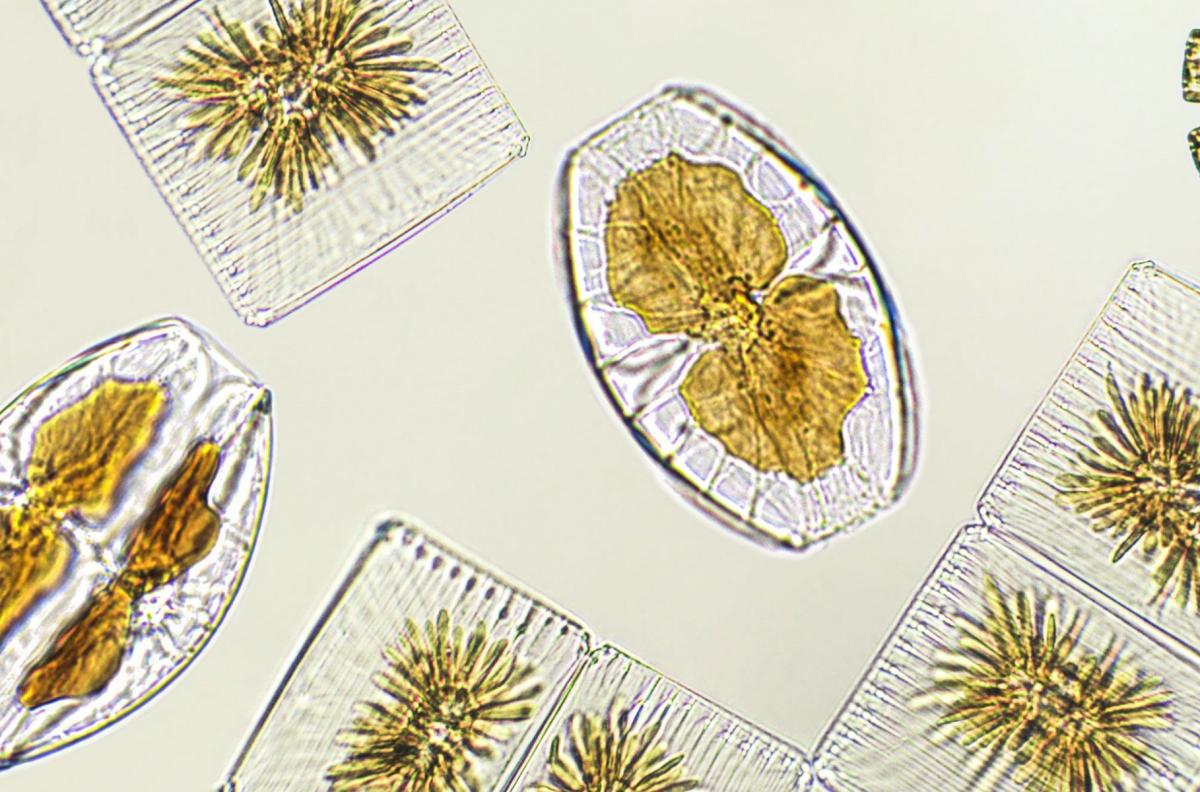
Diatoms are one of the largest groups of phytoplankton, which serve as a foundation of the ocean’s food web. Photo Credit: Elif Bayraktar via iStock
Researchers at UC Santa Barbara are evaluating the effects of increasing the ocean’s alkalinity to boost carbon sequestration. The aim is to speed up the geologic processes that remove carbon from the atmosphere, which are very powerful, but very slow. The team investigated how this would affect two of the ocean’s most numerous and important plankton groups at the bottom of the food chain. Their findings, published in Science Advances, suggest that the plankton would fare well under the treatment, a positive result that encourages more research into this promising proposal.
“As we add CO2 into the atmosphere, we’re acidifying the ocean,” said lead author James Gately, a doctoral student at UC Santa Barbara. “Adding alkalinity is essentially like adding an antacid into the ocean.” Alkaline, or basic, compounds alter the chemistry of seawater, converting CO2 into other carbon compounds, like carbonate and bicarbonate ions. This enables the ocean to absorb more carbon dioxide while reducing the water’s acidity.
In fact, this mechanism forms the basis of the geologic carbon cycle, which recycles carbon between the earth, atmosphere and ocean over long periods of time. “This process typically takes tens to hundreds of thousands of years to occur,” Gately said. “Our aim is to speed this process up.”
The driving question for Gately and his colleagues is how will marine life respond to ocean alkalinity enhancement on large scales? To get at the answer, they looked at how this treatment affected two key groups of plankton: diatoms and coccolithophores.
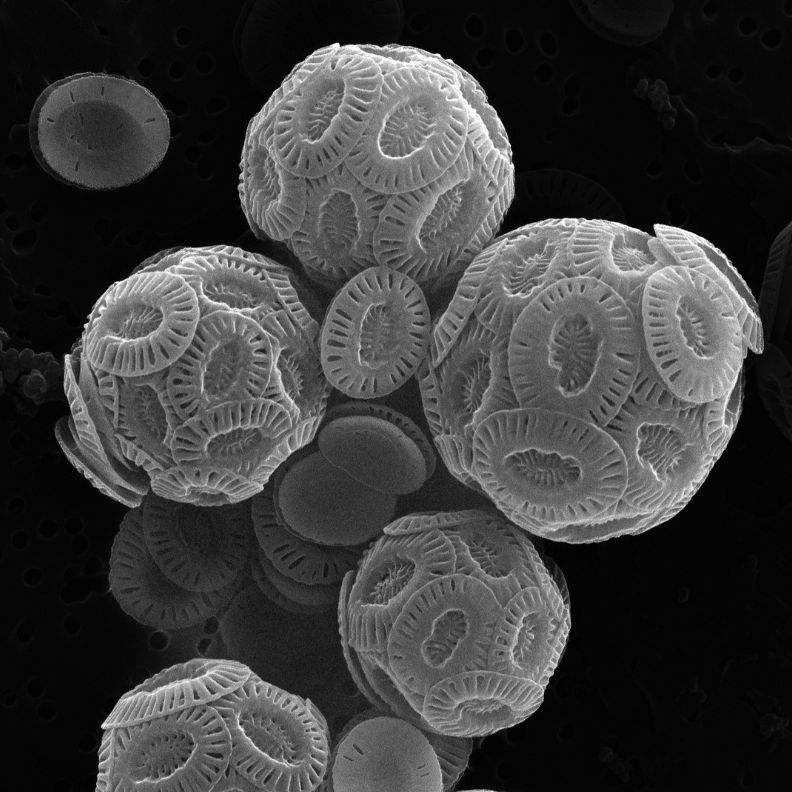
The carbonate shells of ancient coccolithophores are a major component of chalk. Photo Credit: James Gately et al
Both groups are important primary producers, turning sunlight into food and serving as the basis of the ocean’s food chain. “They play a major role in the biological carbon pump, which is essentially the way the oceans lock carbon dioxide away from the atmosphere over millions of years,” said Professor Débora Iglesias-Rodriguez, Gately’s adviser in the Department of Ecology, Evolution, and Marine Biology. These plankton also construct exoskeletons, meaning they move massive amounts of calcium, silica and carbonate around the biosphere.
Annual phytoplankton blooms (e.g., coccolithophores and diatoms) feed small fish and so forth all the way up the food chain. After the blooms, the dead cells fall to the seafloor, creating a carbonate or silica-rich sediment. Over time, this sediment sequesters carbon from the atmosphere that the organisms had taken in through photosynthesis. Eventually, the seafloor sediment can become chert and limestone.
If ocean alkalinity enhancement impacts either of these plankton, the results could be dire.
The team added nutrients and alkalinity to water they collected from the Santa Barbara Channel. Typically, minerals like olivine and various carbonates provide the alkalinity over geologic time, but Gately and his co-authors mimicked this process with other compounds that dissolve and react more quickly. Then they filtered the water to sterilize it before bubbling through air with 420 parts per million carbon dioxide, roughly equivalent to modern atmospheric CO2 concentrations. After a few days, the team added diatoms and coccolithophores they had cultured in the lab.
The alkalinity of the modern ocean is around 2,300 to 2,400 micro-moles per kilogram of water. The scientists ran one trial at 3,000 µmol/kg, simulating long-term alkalinity addition and another at 5,000 µmol/kg to simulate potential hotspots, like a treatment site.
The authors measured a suite of changes to the planktons’ physiology and biochemistry, as well as the chemistry of the seawater. They were particularly curious whether the coccolithophores would increase their calcification, as the treatment would raise the abundance of calcium ions in the water. Ironically, creating calcium carbonate actually produces CO2, even though the compound has carbon and oxygen in it. Over long timescales, the sequestration effects win out, making coccolithophores one of the largest carbon sinks on Earth.
All told, the plankton had a neutral response to the alkalinity treatments, and calcification didn’t change significantly. The cells’ photosynthetic efficiency decreased slightly, but was still well within healthy levels for both treatments. The authors suspect the decrease may be due to reduced availability of micronutrients, like iron.
In fact, the team observed dissolved ions turning into solid compounds, or precipitating, at higher alkalinities. This process can remove nutrients and alkalinity from solution, which could both affect sea life and decrease the efficacy of ocean alkalinity enhancement. The team is already investigating the process in new experiments.
“All told, when we increased alkalinity in the water, the physiology of these organisms did not change,” said Iglesias-Rodriguez. While the results are encouraging, the authors caution against extrapolating out to the ecosystem scale, because responses can vary by species. “Phytoplankton is a good start, but we need to test this in other organisms and ecosystems as well.”
The group has already begun conducting alkalinity enhancement experiments on entire plankton communities under natural nutrient concentrations. They’ll measure the response of individual species as well as the community as a whole. Eventually the team plans to take the research out of the lab and into the field. “It’s very exciting, but we need to operate with caution,” Iglesias-Rodriguez said.
Like many geoengineering proposals, ocean alkalinity enhancement is a controversial topic. “We’re not saying this is a good idea,” Gately said, “we’re trying to determine whether it is or not.”
The problem is that it’s too late to simply rely on reducing our emissions if we want to keep warming under 2 degrees Celsius. Expanding carbon removal to the gigaton scale will require several approaches; ocean alkalinity enhancement is merely one of them. “None of these technologies is a silver bullet for climate change,” Gately said, but the ocean sequesters over an order of magnitude more carbon than the land and atmosphere combined, making ocean-focused approaches attractive.
That said, geoengineering alone can’t solve the problem unless society reduces greenhouse gas emissions. “If we’re in a boat with a hole, we can use a bucket to try to scoop out the water,” Gately added. “But if we don’t plug the hole, we’re going to sink.”